
Clinical trials are scientific studies designed to find ways to improve our ability to prevent, diagnose and treat cancer. To better understand this vital research, we asked Emily Powell, PhD, research clinical operations manager, Parkview Research Center, to answer our questions to delineate how patients may benefit from the new therapies and treatments resulting from this innovative research.
What role do clinical trials play in cancer treatment?
The goal of any clinical trial is to improve our health. This is accomplished by testing new medicines or procedures on people who may benefit from them. However, before these new interventions are tested with patients, they are tested with other mediums, such as animals. These studies are then performed with people only if there is reason to believe the new treatment or test may improve patient care. New treatments are carefully compared to current therapies to determine whether they are safe and effective.
For cancer patients, clinical trials help evaluate new procedures, medications or combinations of treatments in hopes of improving quality of life. Furthermore, these new interventions may be equally or more effective and offer fewer side effects than current standard treatments.
What types of clinical trials are available within the oncology realm?
There are several types of clinical trials, and each is designed to learn more about cancer and/or cancer patients. These categories include:
- Treatment clinical trials: These are the most common. These studies test new modalities intended to shrink tumors more effectively, prevent them from spreading, and have fewer side effects than current therapies.
- Preventative clinical trials: These trials study ways to avert cancer. One example might be reducing alcohol consumption in one group of people, then comparing cancer incidences many years later.
- Screening clinical trials: This type of trial looks for ways to detect cancer earlier than usual.
- Quality of life clinical trials: These trials find better supportive care for cancer patients.
- Registry clinical trials: These do not involve direct intervention to the patient and instead are designed to collect data, often from a patient’s electronic medical record. Scientists can then use that information to make comparisons among large groups of people.
Parkview Research Center has nearly all of these types of trials at any given time. We have a highly-skilled specialized team of doctors and staff who ensure the careful and safe management of these studies in several types of cancer.
Can you describe the various phases of a clinical trial?
Yes, it can best be explained by breaking the phases down into a graphic:
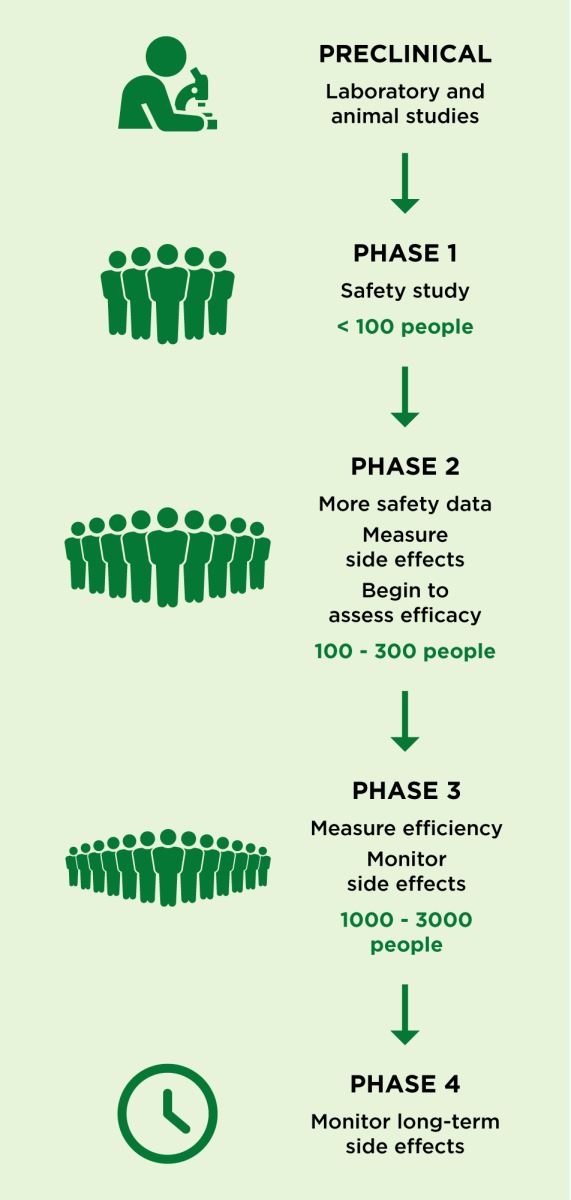
What safety measures are in place for patients who participate in clinical trials?
Study investigators, doctors, safety committees and regulatory agencies such as the Food and Drug Administration (FDA) closely monitor clinical trials. When pharmaceutical companies test new medications in clinical trials, they send specialized personnel called study monitors to carefully examine each study’s conduct and each patient, providing further oversight. Any time there is a potential side effect, no matter how mild it may seem, the research team quickly reports it so that investigators and doctors are aware of any possible adverse reactions.
Does participation in a trial add to a patient’s treatment time or require additional labs and testing?
In some studies, additional doctor visits, labs and/or testing may be required. There may be reduced treatment times in other studies, especially if a study is designed to test a shorter course of radiation therapy medication. It all depends on the study’s scientific question.
When a patient is deciding whether to participate in a clinical trial, the doctor and research nurse will explain any additional time commitments. We find that many patients choose to participate in these studies because the potential benefit outweighs the inconvenience. Patients may also choose to endure the additional time commitment because they are deeply committed to helping future cancer patients and wish to contribute to scientific progress in cancer research.
Is there a cost for participation in a clinical trial?
The Parkview Research Center has a team of highly skilled and specialized billing compliance coordinators who carefully review each protocol before opening a study to our Parkview patients. A patient’s insurance covers most studies, or the study’s sponsor (usually a pharmaceutical company or device manufacturer) pays for any additional costs. In most cases, a patient should incur no further financial commitments due to participating in a clinical trial. However, in very rare instances, a patient may be responsible for some financial commitment. In those cases, the research team is careful to explain this to patients before beginning any trial. The team also estimates any possible dollar amount or costs, so there are no surprises in the future. We do everything in our power to avoid any financial burden because patients who choose to participate in clinical trials are contributing a very valuable service to the field of cancer research.
Who is eligible to participate in a clinical trial?
Anyone who meets the criteria for a clinical trial can participate in one. It doesn’t matter where they live. However, there may be travel costs that the study’s sponsor will not pay, so if the patient doesn’t live nearby, they may have to pay for any airfare, hotel or meals while away from home.
The criteria for oncology clinical trials are often pretty narrow. For example, only patients with a particular type of breast cancer that expresses a specific molecule that the medication interacts with would be eligible. If your cancer does not have this molecule, you would not be eligible to participate in that particular clinical trial. At Parkview, we try to open studies that fit with our patient population, so we have clinical trial options for as many people as possible.
Can providers enroll patients in a clinical trial or can patients self-refer?
Patients are always encouraged to ask their doctors about clinical trials. Our Parkview Cancer Institute physicians and research staff do a fantastic job of looking for all their patients’ options. Every single patient that visits Parkview Cancer Institute gets screened for potential participation in our available clinical trials. Most of our providers are appropriately trained and credentialed for research, so they are ready to enroll their patients in a study.
For more information on Parkview’s Research Center and clinical trials, please visit this page on parkview.com.




